What is Carbon Dioxide Gas?
Carbon Dioxide Gas, Carbon Dioxide (CO2), is a gas with the chemical formula CO2 consisting of one carbon atom (C) and two oxygen atoms (O2). This gas is naturally found in the atmosphere and results from various processes such as animal respiration, plant respiration, and the combustion of organic matter.
As you mentioned, carbon dioxide gas is heavier than air. This is because the carbon atom has a larger mass and combines with two oxygen atoms to form a heavier molecule. This characteristic generally causes carbon dioxide to accumulate at the bottom in the air.
In addition, the presence of carbon dioxide gas in the atmosphere is important because this gas creates a greenhouse effect. When some of the rays from the sun reach the earth's surface, the heat energy reflected from the surface is trapped by greenhouse gases in the atmosphere. Carbon dioxide is also among these gases
What is Carbon Dioxide?
Carbon Dioxide (CO2) is a gas with the chemical formula C(O)=O, containing one carbon atom and two oxygen atoms. It is naturally found in the atmosphere, underground, and in water. The fact that this gas is colorless and odorless means it cannot be directly perceived by humans or other living beings.
An important feature of carbon dioxide is its role in the photosynthesis process of plants. By taking carbon dioxide from the atmosphere, plants use solar energy to produce organic compounds and release oxygen. Photosynthesis is a critical process for plant growth and energy storage.
However, human activities, especially the use of fossil fuels, are increasing carbon dioxide levels in the atmosphere. This increase is one of the main causes of global warming and climate change. High carbon dioxide levels increase the greenhouse effect, trapping heat in the atmosphere and causing climate changes worldwide.
Therefore, it is important to protect the natural balance of carbon dioxide gas and control its increasing levels. Various efforts such as turning to sustainable energy sources, energy efficiency, afforestation, and reducing carbon emissions are implemented with the aim of ensuring the balance of carbon dioxide in the atmosphere.
Properties of Carbon Dioxide Gas
The properties of carbon dioxide gas are as follows:
Weight and State of Matter:
- It is heavier than air and has a molecular weight of 44.01 g/mol.
- It is found in gas form under normal conditions, but it can turn into a solid state at low temperatures. Its solid form is known as "dry ice."
Odor and Color:
- It is an odorless and colorless gas. However, it may have an acidic odor at high concentrations.
Physical Properties:
- It boils at -78 degrees.
- Its density in liquid form is 1.178 kg/liter.
- It has a specific heat of 850 J/(kg·K) at 1 atmosphere pressure and 25 degrees temperature.
- It has an expansion volume of 629 units at 1 atmosphere pressure.
- It has a critical temperature of 31 degrees and a critical pressure of 73.82 bar.
Distribution and Percentage:
- There is 0.035% carbon dioxide gas in the atmosphere.
Chemical Symbol and Usage:
- It is expressed on the periodic table with the chemical symbol CO2.
- It is used in many fields such as gas usage in industrial processes and the carbonation of carbonated beverages in the beverage industry.
Toxic Effects:
- It is toxic in gaseous form and has a particularly suffocating effect. It can cause respiratory distress and loss of consciousness at high concentrations.
What are the Methods for Obtaining Carbon Dioxide Gas?
Carbon dioxide gas can be obtained through various methods. Here are some of these methods:
Obtaining from Natural Sources:
- Carbon dioxide gas emerging from underground sources in volcanic regions can be obtained naturally. Additionally, this gas can be found in abundance at the mouths of craters in the sea. Carbon dioxide gas can also be extracted by opening artesian wells.
Obtaining through the Fermentation Method:
- During the fermentation processes of some bacteria, carbon dioxide gas can be obtained, especially by multiplying cells that produce ethyl alcohol. These bacteria can produce energy without oxygen, and carbon dioxide emerges during this process.
Obtaining through Dry-Ice Production:
- Dry ice, which is the frozen form of carbon dioxide gas, can be produced through various industrial processes. In this process, carbon dioxide gas is liquefied and then this liquid is frozen on a carbonate-based surface to obtain dry ice.
Obtaining by Burning Hydrocarbon Compounds:
- When carbon-containing substances, especially hydrocarbon compounds (such as oil, coal, natural gas), are burned, carbon dioxide gas is released. Therefore, the use of these fuels during energy production and industrial processes is a way of obtaining carbon dioxide gas.
Artificial Production Methods:
- On an industrial scale, carbon dioxide gas can be produced through chemical processes. It usually emerges as a byproduct during ammonia production, gasification plants, and various chemical production processes.
These methods show that carbon dioxide gas can be obtained from different sources and through different processes. It has uses in many areas, from industrial applications to energy production, from fermentation to natural sources.
Where is Carbon Dioxide Gas Used?
Carbon dioxide gas is widely used in various industrial and commercial applications. Here are some usage areas of carbon dioxide gas:
Food Industry:
- In the food sector, carbon dioxide gas is used for storing and transporting foods without spoiling. Especially meat products and fruits are frozen with carbon dioxide gas and can be preserved fresh.
Medicine and Health:
- In the medical field, carbon dioxide gas can be used for anesthesia in surgical procedures. Additionally, carbon dioxide can be used as a cooling agent in surgical laser systems used in surgical areas. Also, it can be used in some surgical applications due to the antibacterial properties of the gas.
Industry:
- In industry, it is used for cooling machines that overheat. Carbon dioxide gas is an effective cooling agent as it passes directly from solid state to gas state.
Greenhouse Gases and Agriculture:
- In greenhouse areas, carbon dioxide can be used to increase the growth of plants. This allows plants to grow faster by accelerating their photosynthesis process.
Cleaning and Metal Processing:
- Carbon dioxide gas can be used in industrial cleaning processes. It is also effective in cleaning metal surfaces.
Fire Extinguishing:
- Carbon dioxide gas is used as an extinguishing agent in fire extinguishing systems, especially in areas where sensitive equipment is located. These systems work by reducing oxygen to suppress the fire.
Airbags:
- Carbon dioxide gas is produced through a rapid reaction inside the airbags used in automobiles. This gas inflates the airbag and reduces potential injuries to the driver and passengers during an accident.
These applications demonstrate the usability of carbon dioxide gas in various industrial, health, and safety fields.

Dangers of Carbon Dioxide Gas
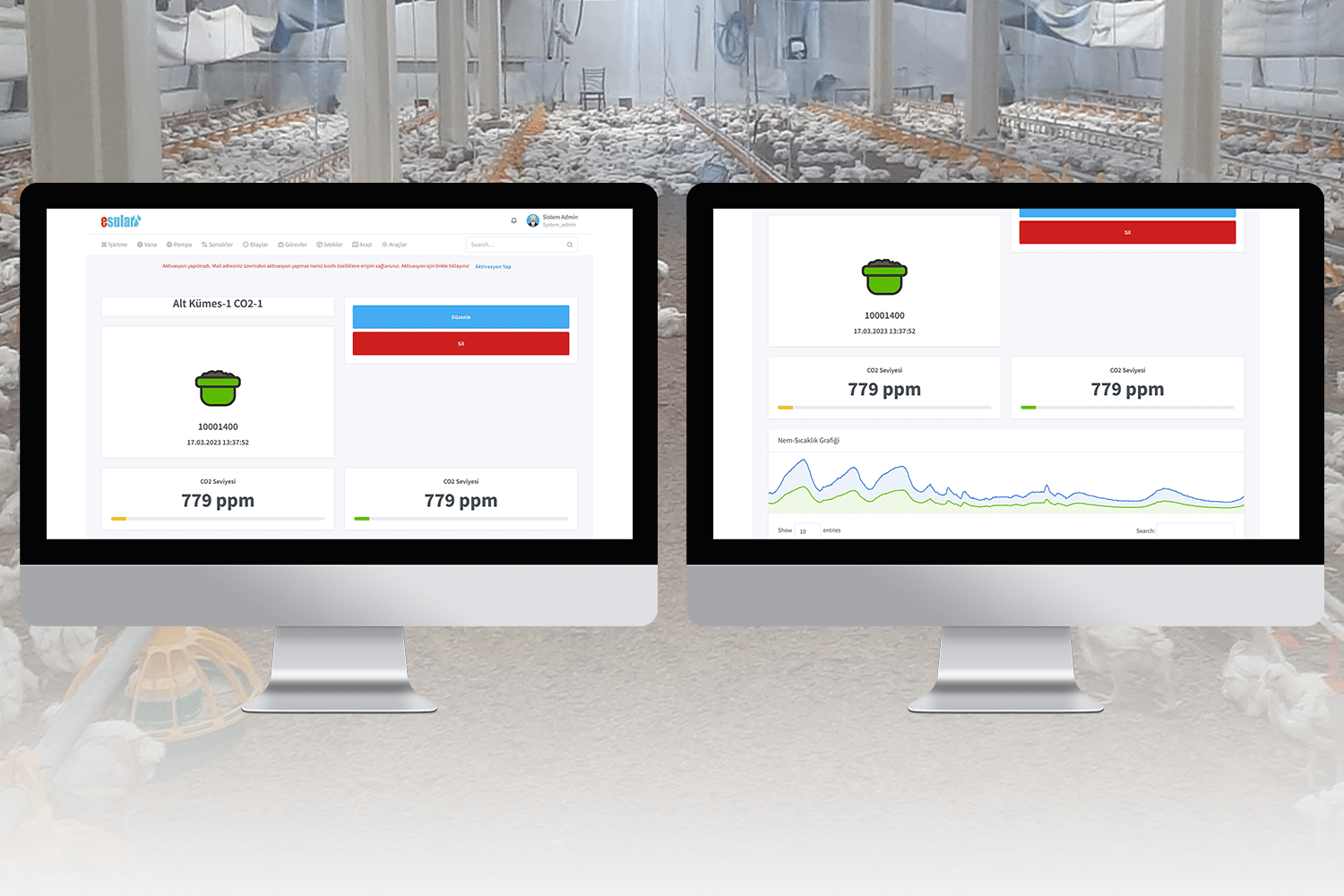
Although carbon dioxide gas is not toxic, it can lead to certain dangers. Due to this gas being heavier than air, it tends to accumulate in enclosed spaces. An excessive amount of carbon dioxide can create a suffocating effect by displacing the oxygen in the environment. The accumulation of carbon dioxide in enclosed spaces can lead to serious health problems by causing respiratory failure and suffocation. Additionally, the fact that carbon dioxide gas is colorless and odorless makes it difficult to detect its presence, and people may not notice this dangerous situation. For this reason, a good ventilation system must be provided in industrial or commercial areas where it is used, and carbon dioxide levels should be monitored regularly. Also, it is important to carefully control the amount of carbon dioxide used in fire extinguishing systems and to use these systems safely.
Use of Carbon Dioxide Gas in the Greenhouse
The use of carbon dioxide gas in the greenhouse environment can increase productivity by supporting the photosynthesis processes of plants and optimizing the atmosphere inside the greenhouse. The advantages of using greenhouse gas can be listed as follows:
Adding carbon dioxide in the greenhouse environment increases the photosynthesis rate of plants. Photosynthesis is a fundamental process through which plants produce energy and oxygen using sunlight and carbon dioxide. With the addition of carbon dioxide, this process accelerates, plants produce more nutrients, and their growth is encouraged.
Especially during the winter months, carbon dioxide levels inside the greenhouse may drop. In this case, it is possible to maintain optimum growth conditions for plants by providing additional carbon dioxide. Higher carbon dioxide levels increase photosynthesis and make plants more resistant to cold weather conditions.
The addition of carbon dioxide allows for obtaining more products by increasing plant density, especially in large greenhouse facilities. This can increase agricultural productivity and optimize the quantity of products.
In some cases, when the carbon dioxide levels in the atmosphere inside the greenhouse are low, the stomata of the plants may close. Stomata are small pores that allow plants to exchange gases. Additional carbon dioxide allows plants to perform this gas exchange more effectively.
However, there are important factors to consider in the use of carbon dioxide. Correct adjustment of the dosage, continuous monitoring of carbon dioxide levels in the atmosphere, and taking safe usage measures ensure that the use of carbon dioxide in the greenhouse is effective and safe.


Yorumlar